- Instrument Management Services
Ensure your surgical instruments are procedure-ready


Reach peak performance with an instrument management partner
Proper management of surgical instruments is critical for maintaining high performance and extending instrument lifespan. Partnering with STERIS Instrument Management Services (IMS) can help you stay procedure-ready while keeping your focus on your patients.
Why STERIS IMS?
STERIS IMS is dedicated to maximizing the lifespan and efficiency of your surgical instruments and equipment. With their expertise, you can ensure that your devices are always ready for any procedure, allowing you to dedicate more time to patient care. Key benefits:
- Extend device lifespan: Reduce malfunctions and prolong the usability of your surgical tools
- Restore performance: Ensure your devices meet the manufacturer’s performance standards
- Comprehensive service: STERIS IMS handles over 2,000 devices from more than 400 manufacturers and 5,000 models
- Loaner program: Access an extensive loaner program to avoid downtime
- Track repairs: Monitor the status of repairs through a user-friendly online portal
- Fast turnaround: Benefit from competitive repair cycles compared to OEMs
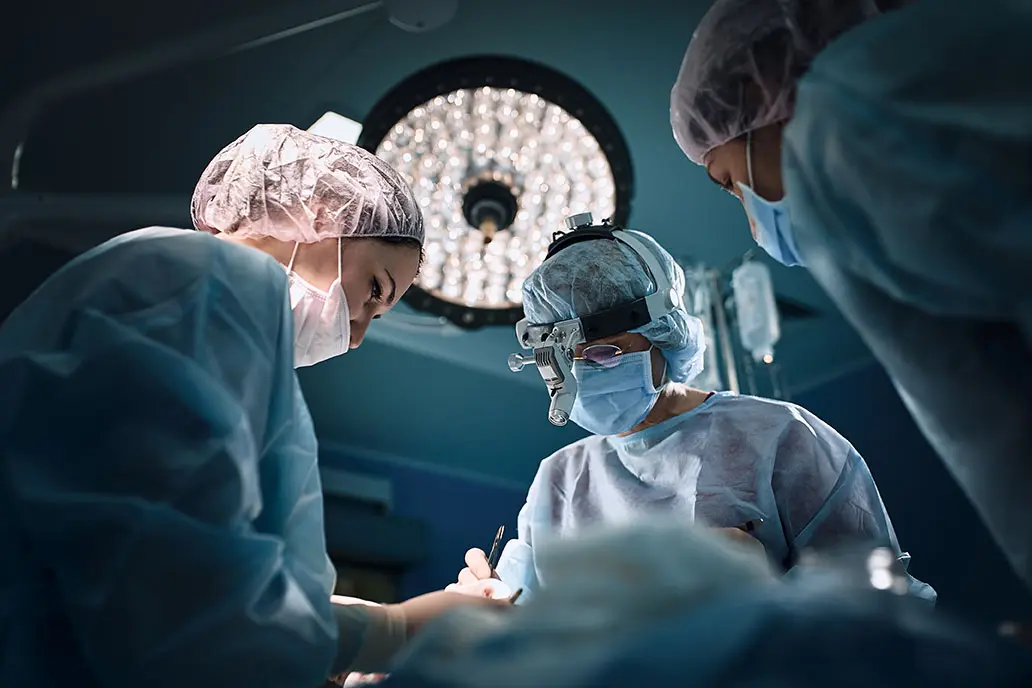
STERIS IMS helps:
Be procedure ready
Mitigate risk
Optimize budgets
We offer a range of services and products to support your practice:
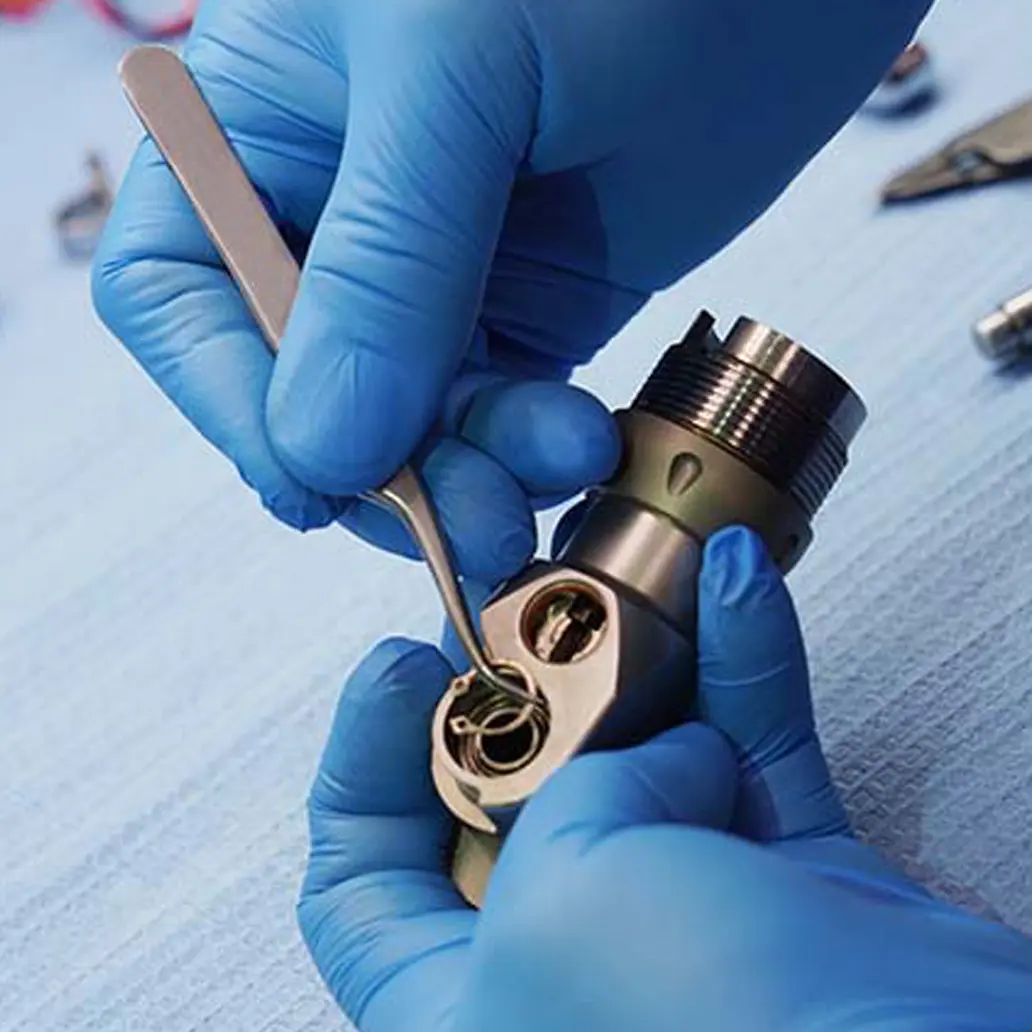
- Flexible endoscope repair
Surgical device repair
- Surgical instrument repair
- Certified pre-owned equipment
STERIS helped save an ASC site over $110,000 (45%) vs. a three-year OEM service agreement. Learn more about how STERIS can offer savings and support.
A nationwide network of experts
5,000+
device models serviced
2,200
repair specialists
4,000
customer faciities

What can STERIS IMS do for you?
Learn more about what best-in class instrument services can do for your practice.
Be advised that information contained herein is intended to serve as a useful reference for informational purposes only and is not compatible clinical information. This information is intended for use only by competent healthcare professionals exercising judgement in providing care. McKesson cannot be held responsible for the continued currency of or for any errors or omissions in the information.
The product information contained in this document, including the product images and additional product materials, was collected from various supplier sources. All product claims and specifications are those of the product suppliers, not McKesson Medical-Surgical or its affiliates (“McKesson”) and have not been independently verified by McKesson. McKesson is not responsible for errors or omissions in the product information. The properties of a product may change or be inaccurate following the posting or printing of the product information in this document, either in the print or online version. Caution should be exercised when using or purchasing any products from McKesson’s online or print documents by closely examining the product packaging and the labeling prior to use. Due to product changes, the information listed in this document is subject to change without notice. This information is placed solely for your convenience in ordering and McKesson disclaims all responsibility for its completeness and accuracy, whether or not the inaccuracy or incompleteness is due to fault or error by McKesson.
© 2024 McKesson Medical-Surgical Inc. All trademarks and registered trademarks are the property of their respective owners. 2024_3377859

