About BD Lab
BD is a global medical technology company that is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. Leading in patient and healthcare worker safety and the technologies that enable medical research and clinical laboratories.

BD Provides Innovative Solutions that Help
Advance cellular studies and genomics
Enhance the diagnosis of infectious disease and cancer
Improve medication management
Promote infection prevention
Equip surgical and interventional procedures
Optimize respiratory care
Support the management of diabetes
Working with organizations around the world to address some of the most challenging global health issues, BD’s associates collaborate closely with customers and partners to help enhance outcomes, lower healthcare delivery costs, increase efficiencies, improve healthcare safety and expand access to health.
Featured product
The BD Veritor™ Plus System: There’s a life behind every test
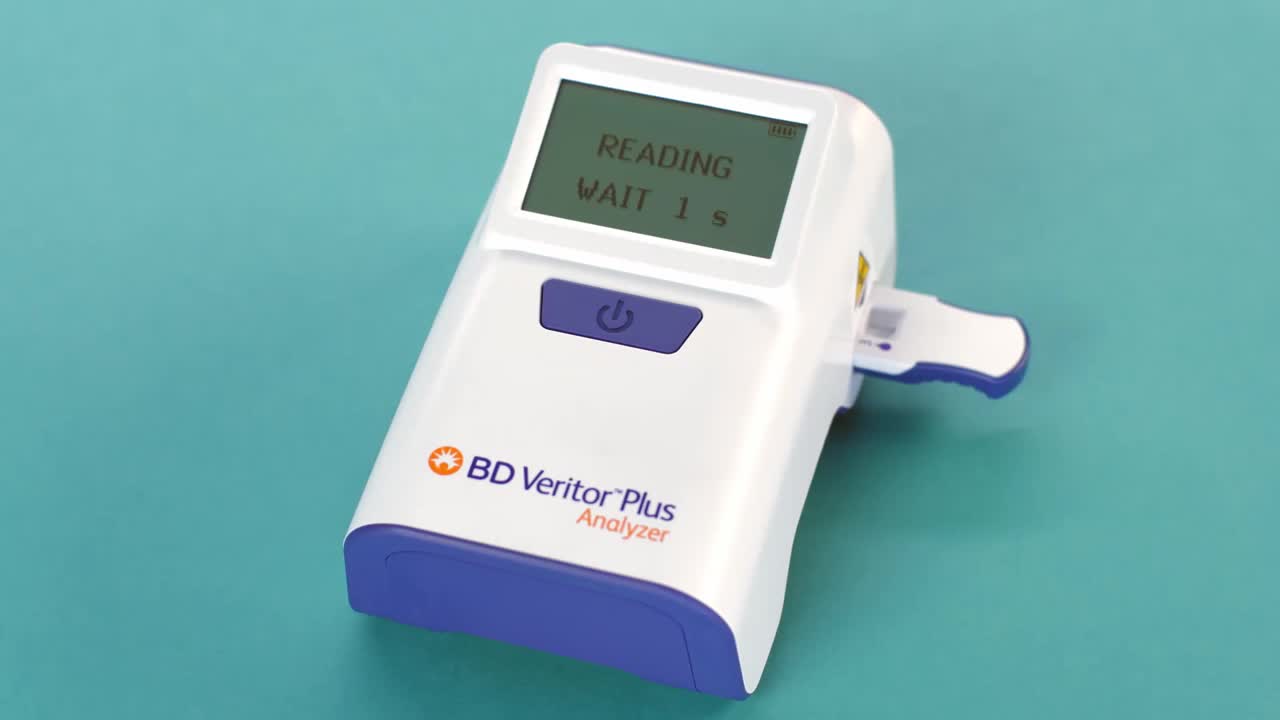
The BD Veritor Plus Analyzer is a digital immunoassay instrument that puts the power of rapid diagnostic testing where you need it; easily, simply and effectively.
The system’s rapid antigen testing assays detect respiratory pathogens, including SARS-CoV-2*, flu A+B, group A strep and respiratory syncytial virus (RSV), delivering reliable diagnostic results at the point-of-care in 15 minutes or less.
- Clear, ambiguous digitally displayed results displayed on screen
- One button operation that automatically identifies what assay is being run
- Two operational modes to adapt to your workflow
- Maintenance free for up to 10,000 tests
- Rechargeable battery for maximum portability
- Optional BD Veritor™ InfoWiFi Module allows barcoded scanning of Specimen ID, Operator ID, and/or kit lot information, with results stored on the device hard drive

SARS-CoV-2 & flu A+B
The BD Veritor Plus System now allows more options on a single assay, allowing you to test for COVID-19, flu A and flu B with just one sample. With multiple testing modes and workflow efficiencies, the analyzer is more versatile than ever. One sample. Three reliable results.
SARS-CoV-2
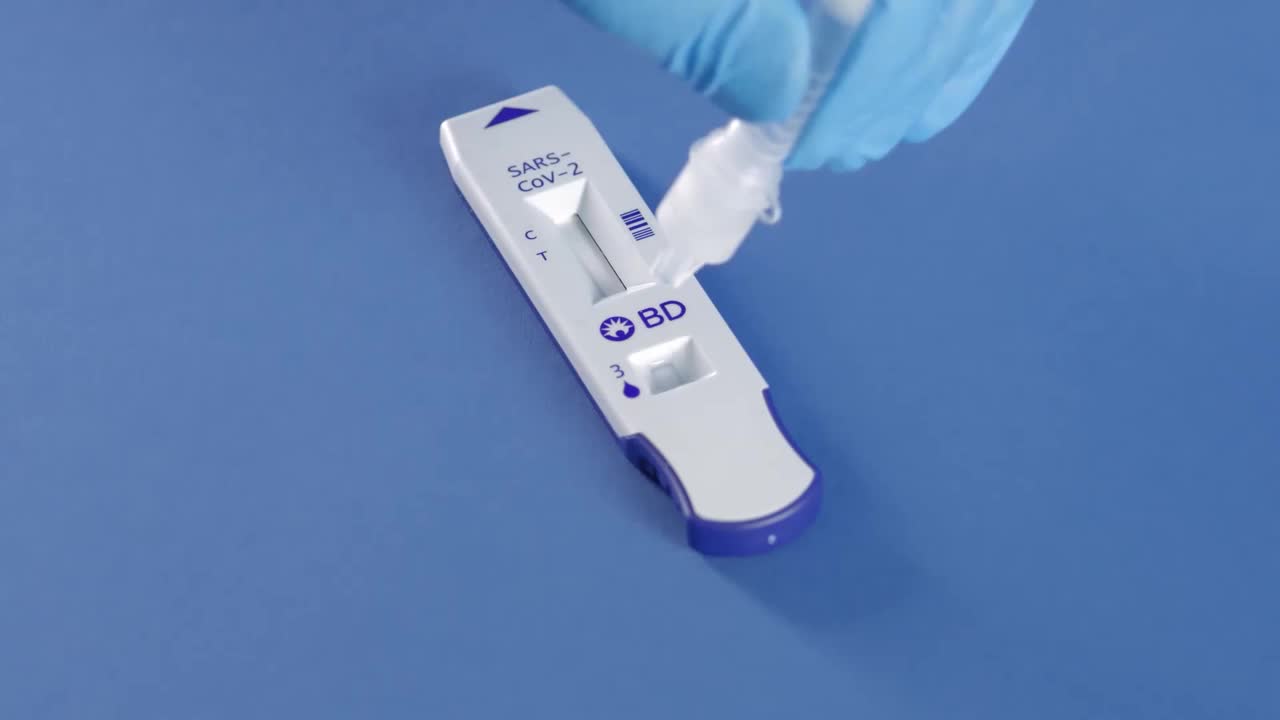
The BD Veritor System for rapid detection of SARS-CoV-2* antigen test detects nucleoproteins from the SARS-CoV-2 virus in 15 minutes at the point-of-care.
Flu A+B
The BD Veritor System for rapid detection of flu A+B is a rapid chromatographic immunoassay. Moderately complex kit also available.
Group A strep
The BD Veritor Plus System for rapid detection of group A strep is a digital immunoassay that quickly, efficiently and reliably detects the presence of group A strep antigen from throat swabs of patients suspected of having group A strep by their healthcare provider.
Respiratory syncytial virus
The BD Veritor Plus System for rapid detection of RSV is a digital immunoassay (DIA) that qualitatively detects RSV fusion protein.
InfoWiFi module
Optional barcode scanner that enables recording of specimen ID, operator ID and kit Llot information for test results. Also enables data download via a USB cable and wireless connection to BD Synapsys™ Informatics Solution.
ImageMover app
Complimentary access to ImageMover companion app for results management and reporting.
Featured products
BD Veritor Plus System
Seasonal promotions for the BD Veritor Plus System
Featured BD Veritor control swabs
BD Veritor Plus System demos
Check out these brief product demos for how to use the BD Veritor Plus System for quick, easy point-of-care rapid testing
Rapid COVID-19 (SARS-CoV-2) + flu A+B testing
Rapid COVID-19 (SARS-CoV-2) testing
Rapid flu A+B testing
*Emergency Use Authorization information for the BD Veritor™ SARS-CoV-2 and SARS-CoV-2 & Flu A+B assays:
• These products have not been FDA cleared or approved; but have been authorized by FDA under EUA for use by authorized laboratories
• The BD Veritor™ System for Rapid Detection of SARS-CoV-2 has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; the BD Veritor™ System for Rapid Detection of SARS-CoV-2 & Flu A+B has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens; and,
• The emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Be advised that information contained herein is intended to serve as a useful reference for informational purposes only and is not complete clinical information. This information is intended for use only by competent healthcare professionals exercising judgment in providing care. McKesson cannot be held responsible for the continued currency of or for any errors or omissions in the information.
The product information contained in this document, including the product images and additional product materials, was collected from various supplier sources. All product claims and specifications are those of the product suppliers and have not been independently verified by McKesson Medical-Surgical or its affiliates (“McKesson”). McKesson is not responsible for errors or omissions in the product information. The properties of a product may change or be inaccurate following the posting or printing of the product information in the document, either in the print or online version. Caution should be exercised when using or purchasing any products from McKesson’s online or print documents by closely examining the product packaging and the labeling prior to use. Due to product changes, information listed in this document is subject to change without notice. This information is placed solely for your convenience in ordering and McKesson disclaims all responsibility for its completeness and accuracy, whether or not the inaccuracy or incompleteness is due to fault or error by McKesson.
©2024 McKesson Medical-Surgical Inc. All trademarks and registered trademarks are the property of their respective owners.

















